Dynamic Digital Radiography Receives FDA Clearance
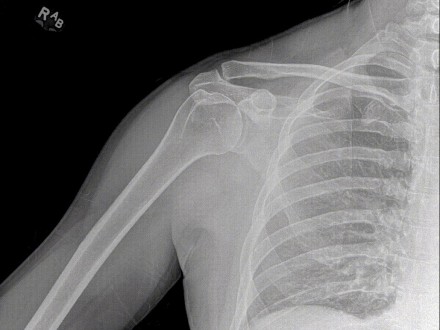
Konica Minolta Healthcare Americas, Inc., announces that the transformative technology introduced at RSNA 2018, Dynamic Digital Radiography (DDR), has received 510(k) clearance from the US Food and Drug Administration. DDR represents the next evolution in X-ray imaging with the ability to capture movement in a single exam and is a fundamental change in the way clinicians can utilize radiography.
